|
Qufu HiTech Trading Company
|
Eye Surgey Medical Sodium Hyaluronate Gel For Ocular surgery
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
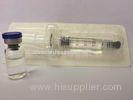
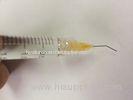
Hyaluronic Acid Injec
Hyaluronic Acid Injections Medical Sodium Hyaluronate Gel For Ocular surgery
Specification
|
Test Items |
Standard |
|
Appearance |
Colorless liquid |
|
Appearance of Solution (A600nm) |
<0.01% |
|
ID-A |
Infrared Absorption Spectrophotometry |
|
ID-B |
Reaction of Sodium |
|
PH |
6.5 |
|
Molecular weight |
1. 8 X 106 |
|
Protein |
0.01% |
|
Nucleic Acids (A260nm) |
<0.5 |
|
Chlorides |
.2 |
|
Packaging: |
1ml/syringe;2ml/syringe;3ml/syringe |
|
Concentration: |
10mg/ml;15mg/ml;18mg/ml;20mg/ml;25mg/ml;30mg/ml |
|
Storage: |
Store between 2 and 20,Do not Freeze. Relative humidity no more than 80%, well ventilated and clean, no corrosive gas, protect from light. Do not uses after expiry date |

Description:
Sodium Hyaluronate, which is a polymer polysaccharides of biological materials formed by N-acetyl glucosamine and glucuronic acid through iterative connected, has a high degree of viscoelasticity, plasticity, permeability and good biocompatibility. This product is manufactured with raw material of comb based on the process of fine biochemical technology, separation, purification and refining. The gel contains 1% HMW of sodium hyaluronate and physiological buffer. This product, which is a colorless, odorless, sterile, pyrogen free transparent viscous liquid, adopts the aseptic processing technology to filter sterilization.

Constitute & function :
1. Moist eyeball surface during surgery, and prevent epithelium from dry. The function of this product is much better than physiological saline or equilibrium liquid.
2. Inject this product under bulbar conjunctiva to separate conjunctival flap, and minimized the post-operative sub-conjunctive-fibrosis during glaucoma surgery.
3. Form protective layer to reduce the damage of device or intraocular lens implantation to endothelial cell, and reduce loss rate of endothelial cell.
4. Convenience to operation, such as extraction of foreign body in anterior chamber, intraocular lens implantation